
BIOLOGY REVISION: THE EXTRACELLULAR MATRIX
First thing to know… it is very complex! So, let’s break it down a bit to make it a little easier to understand.
The extracellular matrix (ECM)
The material that surrounds animal cells
Produces a variety of structures (like bone, tendons, shells of molluscs)
Not just a structural role, it can regulate the behaviour of its resident cells
Abundant in connective tissue (CT) both loose and dense
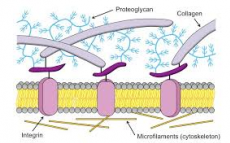
Spaces around cells are filled with hydrated polysaccharide glycosaminoglycans often linked to proteins to form a proteoglycan gel
It influences the survival development, migration, shape, proliferation and function of the cells within it. Molecules of the ECM are produced locally (for example secreted by fibroblasts in loose connective tissues, by osteoblasts and chondroblasts in bone/cartilage respectively).
Glycosaminoglycan (GAGs)
Chains are repeated units of negatively charge disaccharides that form linear chains, and that attract cations (sodium) causing large amounts of water to be sucked into the matrix
Most can be linked to proteins to form proteoglycans
All GAGs and proteoglycans are synthesised in, and secreted by, cells in the ECM
GAGs and Proteoglycans
Varied nature means that pore sizes in the gel, and charge densities vary. This influences not only turgor(water), but also what cells may pass through the ECM
PGs can bind signalling molecules enhancing or inhibiting their activity to affect nearby cell proliferation slide 11
Like the GAGs and proteoglycans, these proteins are secreted by cells in the ECM (the secretory pathway is very active in these cells). Collagens/elastin give strength and resilience, and can be anchored by sticky fibronectin/laminin to the integrins of cells.
Fibroblasts in connective tissue
Showing cells (fibroblasts) and ECM proteins
It is mainly collagen fibres that are visible because the hydrated gel of GAGs and proteoglycans has been removed by enzyme and acid treatments in preparation for microscopy
Collagens
The most abundant proteins found in the animal kingdom
there are ~25 distinct alpha chains used in various combinations give 20 types of collagen.
There are 46 different collagen genes dispersed throughout the human genome
the main types in connective tissues are types I, II, III, V and XI – the fibrillar collagens. Each collagen trimer can cross-link to others to form fibrils
Types IX and XII are the fibril-associated collagens
Type IV forms a mesh in basal laminae
are trimeric (B) (3 chains of the same type)
characterised by a stiff triple stranded helical structure with lots of H- bonds between the 3 subunits
each hydrophobic polypeptide (α chain) is ~1,000 amino acids
they are predominantly synthesized by fibroblasts however epithelial cells are sometimes responsible
Fibrillar Collagens
made in cells as precursors (procollagen) with N- and C-terminal propetides that are not present in the mature extracellular collagen
these proteins undergo numerous modifications from their site of folding in the ER lumen to their deposition in the extracellular matrix
image- http://ib.bioninja.com.au/standard-level/topic-1-cell-biology/13-membrane-structure/extracellular-matrix.html

0 Comment:
Be the first one to comment on this article.