

All lipids contain the elements carbon, hydrogen and oxygen. They are useful energy storage molecules. Lipids contain substances such as waxes, steroids, fats, and phospholipids.
Triglycerides
Triglycerides are a type of lipid and are the main constituents of natural fats and oils. They are macromolecules which are complex and have a relatively large molecular mass. The structure consists if one glycerol molecule attached to three fatty acid chains. Fatty acid chains are ‘tail-like’ structures composed of hydrocarbons. As you can gather from the name, hydrocarbons are compounds that only contain carbon and hydrogen. These tails are hydrophobic meaning they repel water molecules. The property of being hydrophobic also means that they make lipid molecules insoluble in water. Insolubility is the term used to describe the incapability to be dissolved. Triglyceride fatty acid chains have a basic structure, containing a carboxylic group attached to an ‘R’ group. ‘R’ groups mean variable, therefore different fatty acid chains will have different R groups. Examples of R groups could be a simple hydrogen atom.
A glycerol molecule is composed of three carbon atoms, hydrogen and oxygen. Glycerol contains an ‘OH’ (hydroxyl group) which reacts with the OH group on the fatty acid chains. Three OH groups from glycerol react with all fatty acid chains in a condensation reaction to produce a triglyceride and three water molecules. The synthesis of triglycerides is called esterification, in this process an ester bond will form where the water molecule is removed. The reaction is reversible, thus triglycerides can be broken down back into glycerol and fatty acids through the addition of a water molecule. Adding a water molecule to break a molecule is called hydrolysis. Triglycerides can store both energy and carbon.
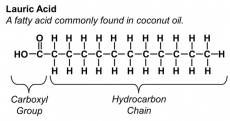
There are two types of fatty acids; saturated and unsaturated. In saturated fatty acids, the bonds between carbon atoms are all single. The general formula for saturated fatty acids is CnH2n+2. Unsaturated fatty acids have at least one double bond. The double bond causes the structure to kink, therefore the molecules in saturated fats are packed more tightly that unsaturated. This makes unsaturated fats healthier as less fat is in a given volume. The general formula for unsaturated fatty acids is CnH2n.
Phospholipids
Phospholipids are another type of macromolecule with a similar structure to triglycerides. The difference is, rather than three fatty acid chains, there is two. Furthermore, attached to the glycerol molecule is a phosphate group. The phosphate group is hydrophilic (meaning it attracts water) but the fatty acid tails are still hydrophobic. You may recognise the word phospholipid, because the cell surface membrane is composed of the phospholipid bilayer. Inside the bilayer is another type of lipid; called cholesterol.
Cholesterol
Cholesterol molecules help to strengthen the cell membrane by making it less fluid and more rigid and compact. Cholesterol has a hydrocarbon ring structure attached to a hydrocarbon tail. The ring structure has a polar hydroxyl group attached to it. Cholesterol is very small and has a flattened shape making it suitable for its function.
Image Credits:
https://fthmb.tqn.com/pNWCIIx6evb1ebJDwnltQzR14NQ=/768x0/filters:no_upscale()/oil_and_water-56a09afd3df78cafdaa32d18.jpg
https://dlc.dcccd.edu/images/biology/lesson3/lauric_acid_structural_model.jpg

0 Comment:
Be the first one to comment on this article.